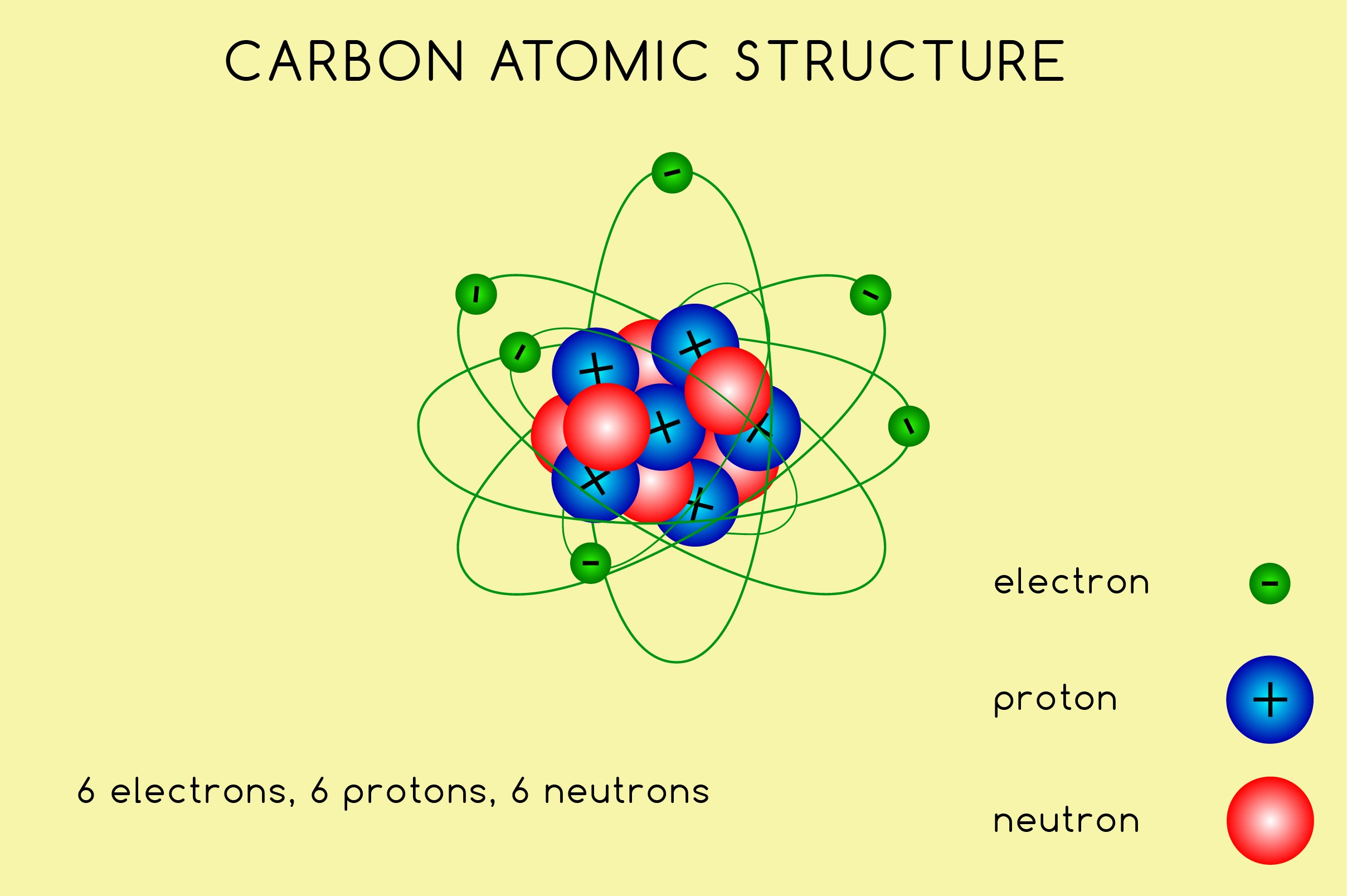
A Carbon Atom Is Most Likely To Form - Web for example, each atom of a group 4a (14) element has four electrons in its outermost shell and therefore requires. In most cases, carbon shares electrons with other atoms. Web as we will see below, the periodic table organizes elements in a way that reflects their number and pattern of electrons,. Carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. Web atoms share electrons and form covalent bonds to satisfy the octet rule. The periodic table and trends in valence electrons can. Web the most common type of bond formed by carbon is a covalent bond. Web one carbon atom forms four covalent bonds with four hydrogen atoms by sharing a pair of electrons between itself and each.
Web atoms share electrons and form covalent bonds to satisfy the octet rule. In most cases, carbon shares electrons with other atoms. Web one carbon atom forms four covalent bonds with four hydrogen atoms by sharing a pair of electrons between itself and each. The periodic table and trends in valence electrons can. Carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. Web as we will see below, the periodic table organizes elements in a way that reflects their number and pattern of electrons,. Web for example, each atom of a group 4a (14) element has four electrons in its outermost shell and therefore requires. Web the most common type of bond formed by carbon is a covalent bond.





![[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds](https://i2.wp.com/d1avenlh0i1xmr.cloudfront.net/small/65382287-b5ac-4ea6-8a57-1f5a107b172c/a-carbon-atom---teachoo.jpg)