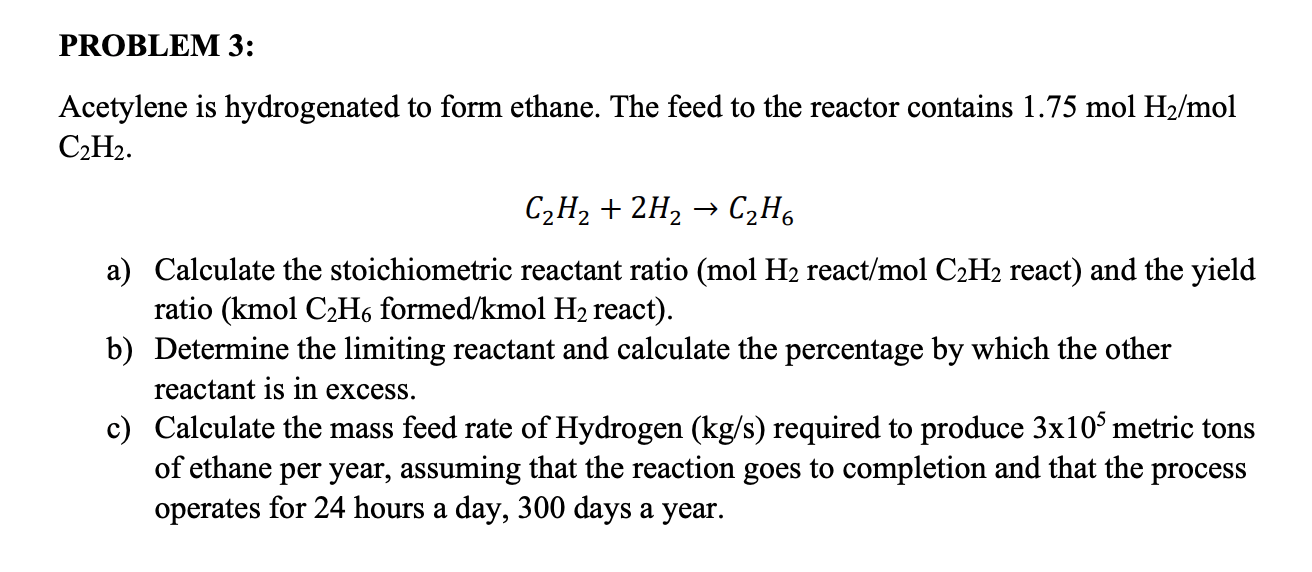
Acetylene Is Hydrogenated To Form Ethane - Acetylene is hydrogenated to form ethane. Web acetylene is hydrogenated to form ethane. The feed to the reactor contains 1.50 mol h2/mol c2h2. What is the percent yield of c2h6 for this. Web the limiting reactant in the hydrogenation of acetylene to ethane, with feed containing 1.4 mol h2/mol c2h2, is. Web when 28.0 grams of acetylene reacts with hydrogen, 24.5 grams of ethane is produced. The feed to the reactor contains 1.50 mol h2 /mol c2h2.
Web acetylene is hydrogenated to form ethane. Acetylene is hydrogenated to form ethane. Web the limiting reactant in the hydrogenation of acetylene to ethane, with feed containing 1.4 mol h2/mol c2h2, is. What is the percent yield of c2h6 for this. Web when 28.0 grams of acetylene reacts with hydrogen, 24.5 grams of ethane is produced. The feed to the reactor contains 1.50 mol h2 /mol c2h2. The feed to the reactor contains 1.50 mol h2/mol c2h2.