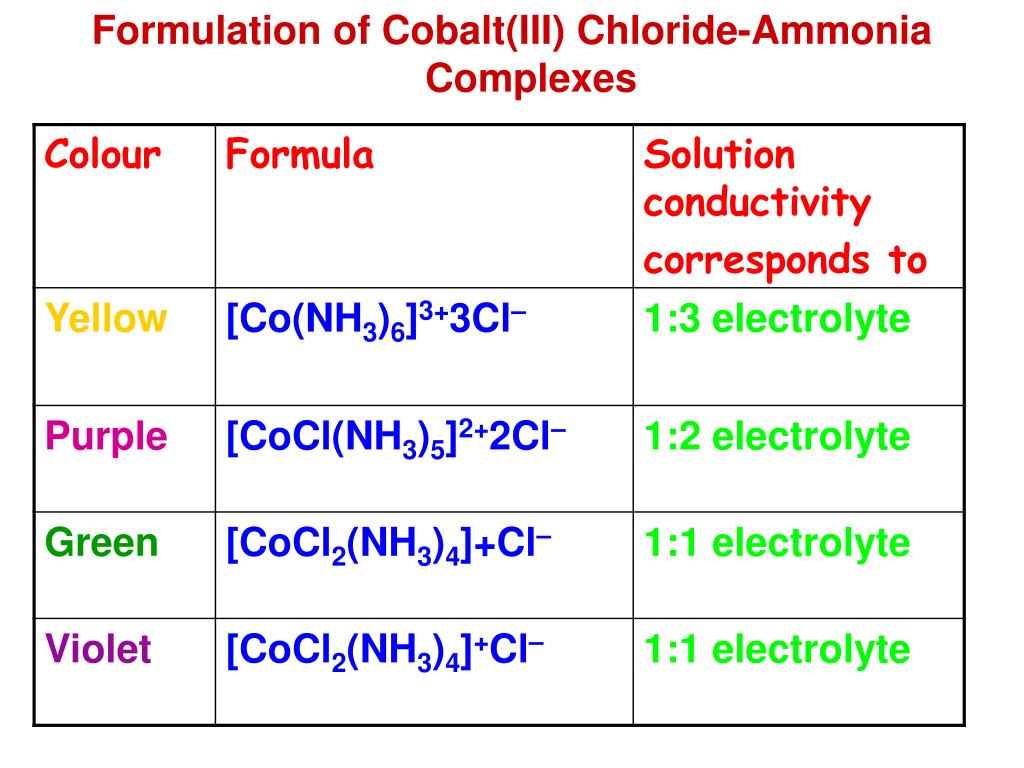
Cobalt Ions Form Complex Ions With Water And Chloride - Web hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the cobalt ion. The addition of hydrochloric acid to the solution provides a high concentration of chloride. Web a solution of cobalt(ii) ion in water is pink, the color of the complex ion formed between co2+ ions and water molecules. A white background will help to show the colour. On the front cover, the pink colour in the test tube comes from cobalt (ii) ions in water, co (h 2 o) 62+. Web co 2+ + 6 h 2 o → [co (h 2 o) 6] 2+. Web however cobalt does not react with water that is at room temperature. Web pink cobalt species + chloride ions ⇌ blue cobalt species + water molecules. The simplest ion that cobalt forms in solution is the pink. Web due to the common ion effect, we might expect a salt such as agcl to be much less soluble in a concentrated solution of kcl.
On the front cover, the pink colour in the test tube comes from cobalt (ii) ions in water, co (h 2 o) 62+. Web however cobalt does not react with water that is at room temperature. Web pink cobalt species + chloride ions ⇌ blue cobalt species + water molecules. Web due to the common ion effect, we might expect a salt such as agcl to be much less soluble in a concentrated solution of kcl. Web hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the cobalt ion. The addition of hydrochloric acid to the solution provides a high concentration of chloride. The simplest ion that cobalt forms in solution is the pink. Web co 2+ + 6 h 2 o → [co (h 2 o) 6] 2+. Web a solution of cobalt(ii) ion in water is pink, the color of the complex ion formed between co2+ ions and water molecules. A white background will help to show the colour.