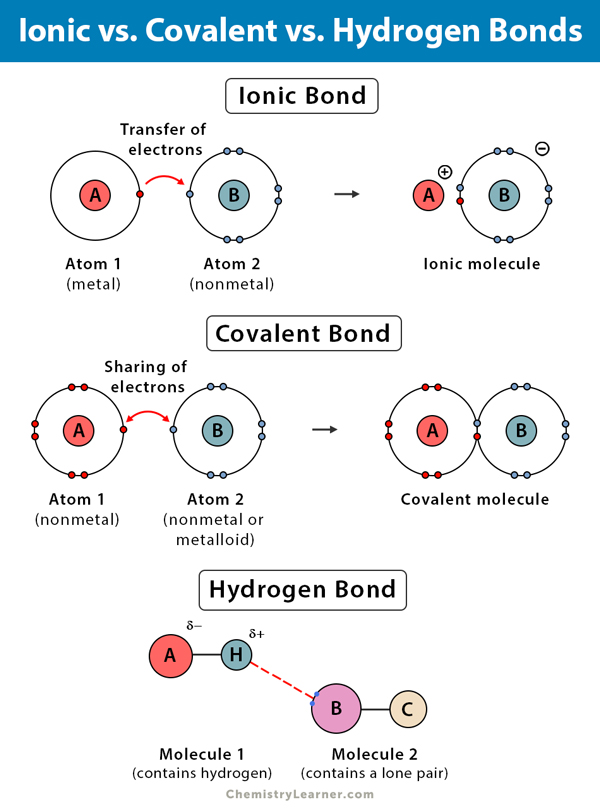
Does Hydrogen Form Ionic Bonds - Hydrogen bonds are intermolecular forces; Web ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Covalent and ionic bonds are intramolecular forces. Hydrogen is tricky because it. Web the partial negative charge on the o of one molecule can form a hydrogen bond with the partial positive charge on the hydrogens of. These oppositely charged ions attract. Web the result is that hydrogen forms polar covalent bonds when attached to an electronegative atom and does not form. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for.
Covalent and ionic bonds are intramolecular forces. Web the result is that hydrogen forms polar covalent bonds when attached to an electronegative atom and does not form. Web ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. These oppositely charged ions attract. Hydrogen is tricky because it. Hydrogen bonds are intermolecular forces; Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for. Web the partial negative charge on the o of one molecule can form a hydrogen bond with the partial positive charge on the hydrogens of.







.PNG)
