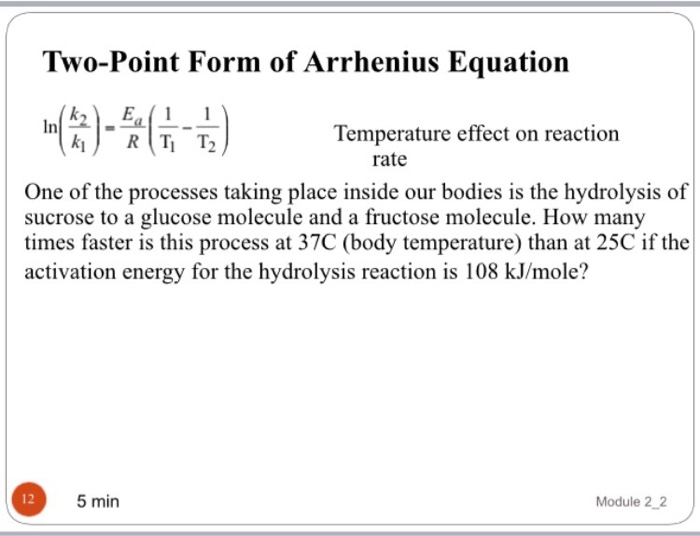
Two Point Form Of Arrhenius Equation - K = − e a r ( 1 t) + ln. For this purpose, the rate constant is measured for two different temperatures t 1 and t 2. K 2 k 1 = e a r ( 1 t 1 − 1 t 2) Web the arrhenius equation gives the dependence of the rate constant of a chemical reaction on the absolute temperature as. Lnk = −ea r ( 1 t)+lna ln. 1.1k views 3 years ago general chemistry 2. Ln k2 k1 = ea r ( 1 t 1 − 1 t 2) ln.
1.1k views 3 years ago general chemistry 2. Lnk = −ea r ( 1 t)+lna ln. K 2 k 1 = e a r ( 1 t 1 − 1 t 2) Ln k2 k1 = ea r ( 1 t 1 − 1 t 2) ln. For this purpose, the rate constant is measured for two different temperatures t 1 and t 2. K = − e a r ( 1 t) + ln. Web the arrhenius equation gives the dependence of the rate constant of a chemical reaction on the absolute temperature as.