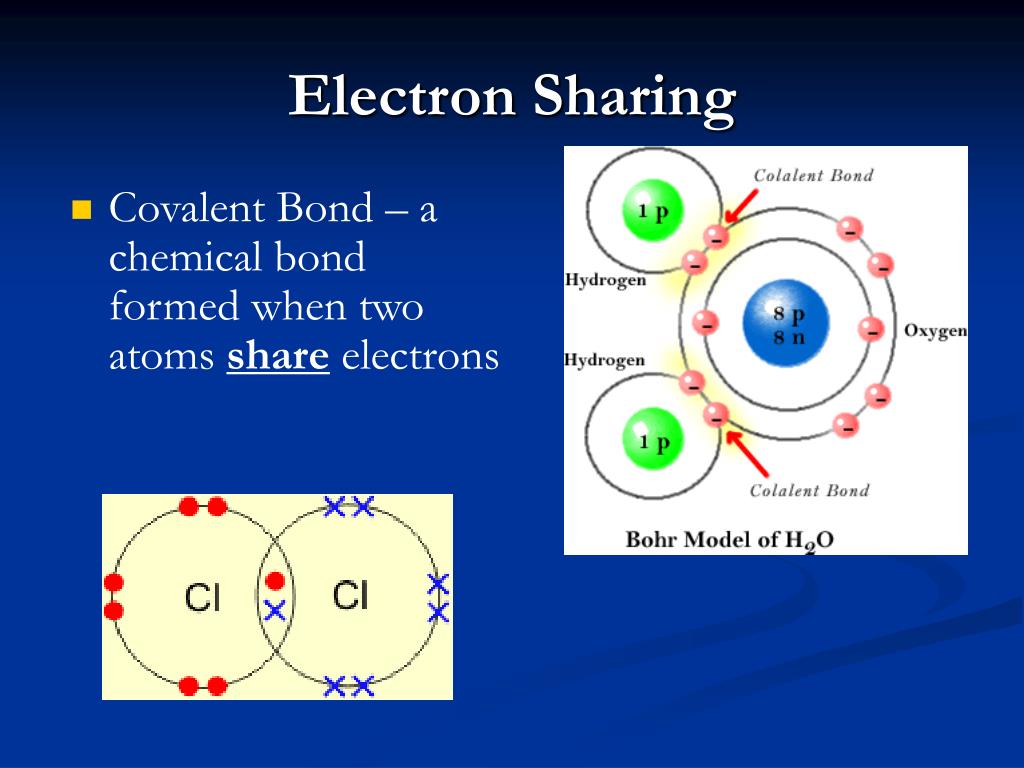
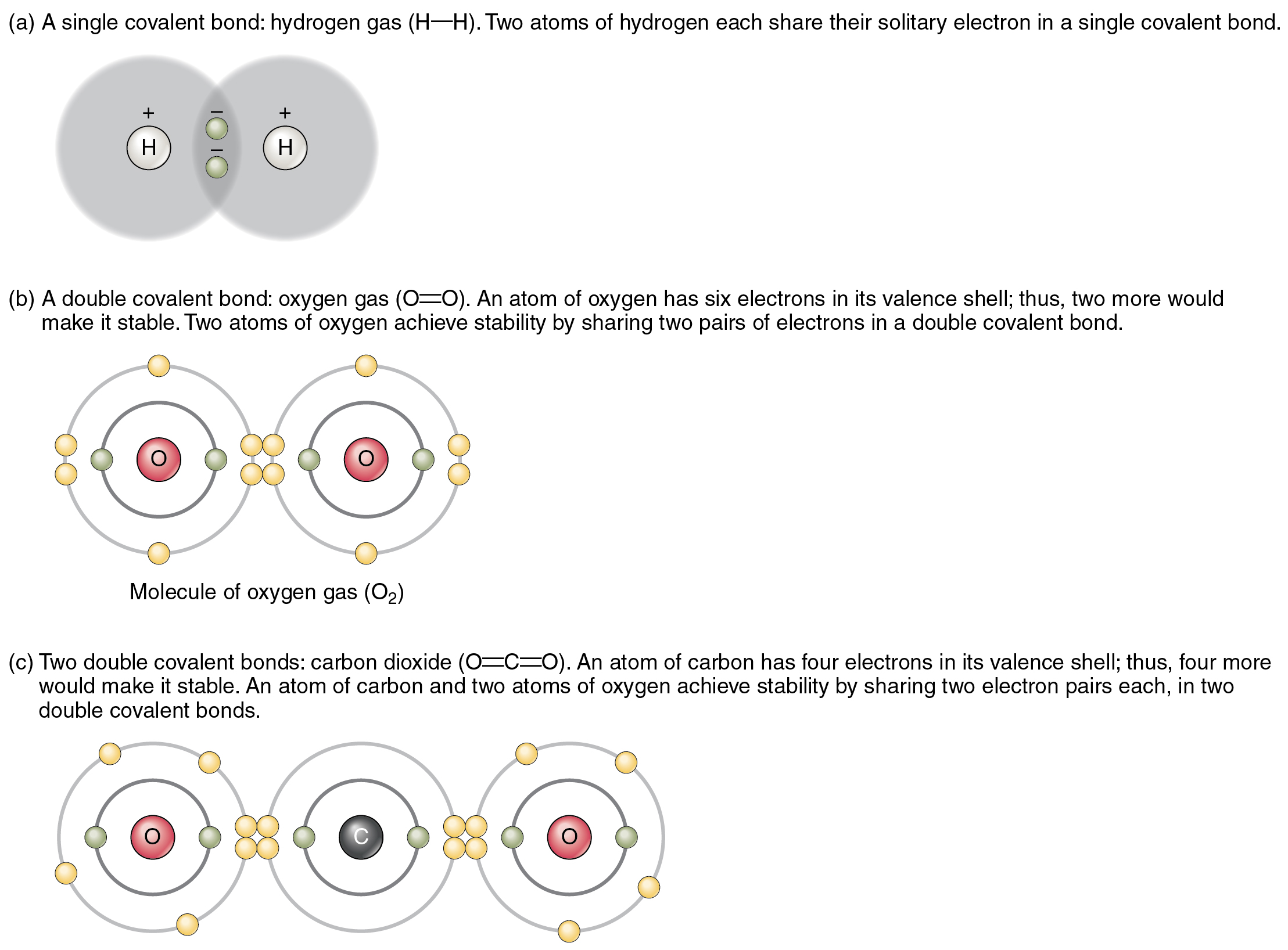
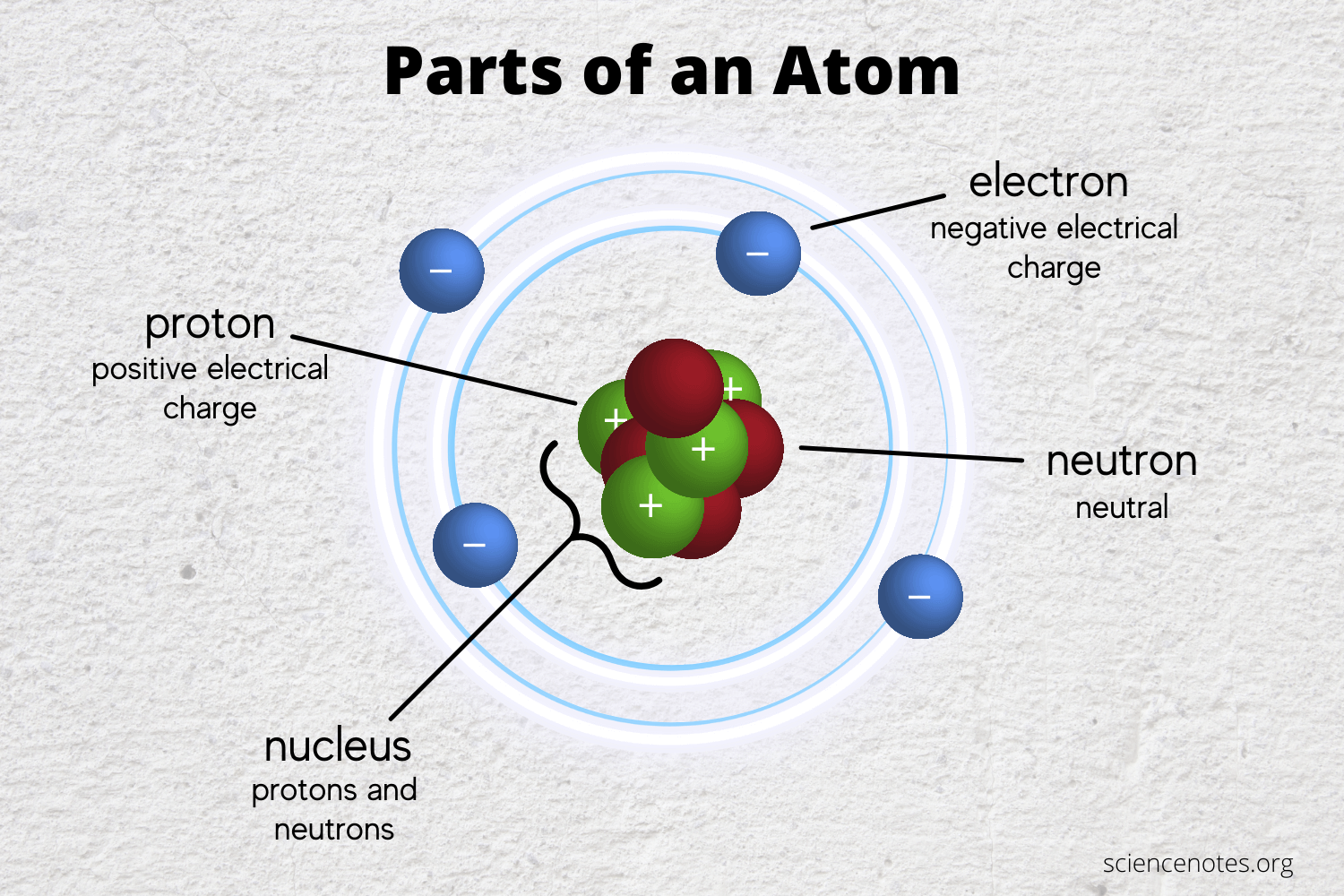
What Do Atoms Form When They Share Electron Pairs - It is this sharing of electrons that we refer to as a chemical bond, or more specifically, as a covalent bond, so named because the bond acts to satisfy the valence of both atoms. Web each atom donates a single electron to the electron pair which is shared. Web a covalent bond is formed when two atoms share electron pairs. Atoms share the same number of pairs needed to fill their valence shell, usually with eight. Web covalent bonds involve shared electron pairs between atoms. Web covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms have identical or fairly similar ionization energies and electron affinities). In a covalent bond, the stability of the bond comes from the shared electrostatic attraction between the two positively charged atomic nuclei and the shared, negatively charged electrons between them. The two atoms are thus held together by the need to share the electron pair. Each atom contributes one electron to each shared pair, and effectively gains an additional electron from the shared pair.
Web covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms have identical or fairly similar ionization energies and electron affinities). Web covalent bonds involve shared electron pairs between atoms. It is this sharing of electrons that we refer to as a chemical bond, or more specifically, as a covalent bond, so named because the bond acts to satisfy the valence of both atoms. Each atom contributes one electron to each shared pair, and effectively gains an additional electron from the shared pair. Atoms share the same number of pairs needed to fill their valence shell, usually with eight. In a covalent bond, the stability of the bond comes from the shared electrostatic attraction between the two positively charged atomic nuclei and the shared, negatively charged electrons between them. Web each atom donates a single electron to the electron pair which is shared. The two atoms are thus held together by the need to share the electron pair. Web a covalent bond is formed when two atoms share electron pairs.




![[ANSWERED] What do atoms form when they equally share electron](https://i2.wp.com/media.kunduz.com/media/sug-question-candidate/20220511034952883386-4536923.jpg?h=512)