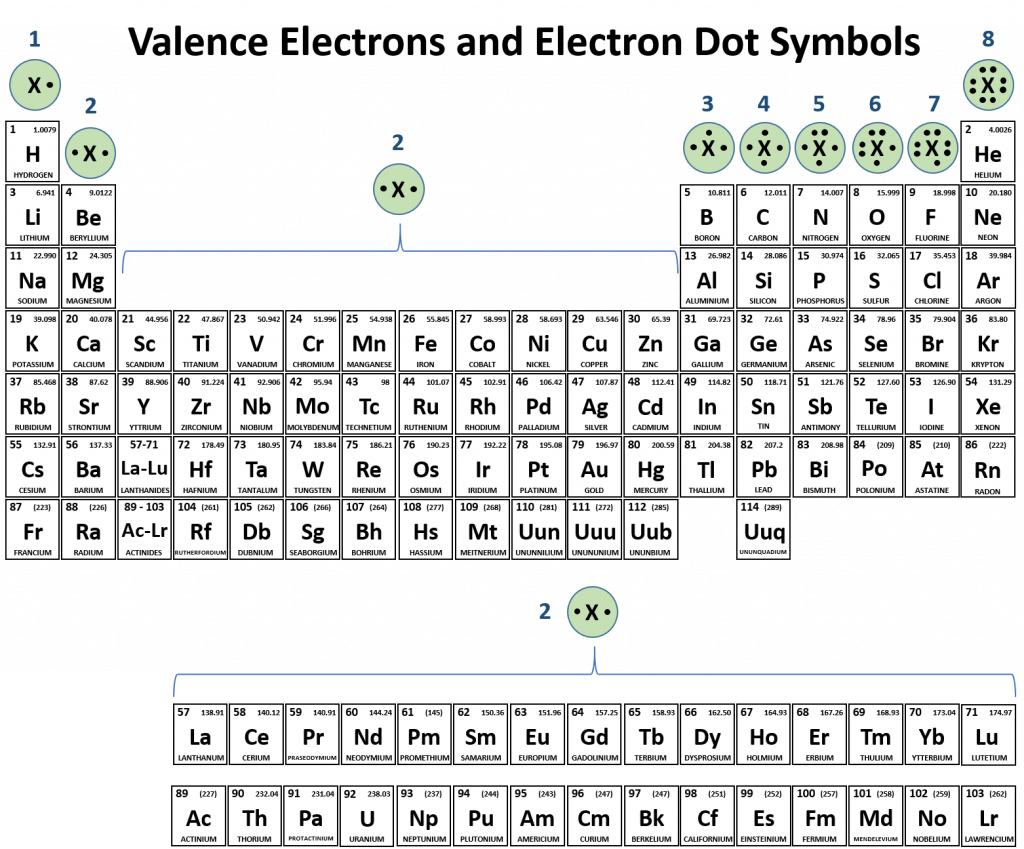
Which Group Tends Not To Form Ions Or React - Group 2 elements form 2+ ions, and so on. That is, group 1 elements form 1+ ions; Web it is important to note, however, that the formula for an ionic compound does not represent the physical arrangement of its ions. Click the card to flip 👆. Click the card to flip 👆. Group 16 elements (two groups left) form 2− ions, and so on. This trend can be used as a guide in many cases, but its predictive value decreases when moving toward the center of the periodic table. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; Which group tends to form 1+ ions? It is incorrect to refer to a sodium chloride (nacl) “molecule” because there is not a single ionic bond, per se, between any specific pair of sodium and chloride ions.
Which group tends to form 1+ ions? Click the card to flip 👆. Group 16 elements (two groups left) form 2− ions, and so on. Group 2 elements form 2+ ions, and so on. This trend can be used as a guide in many cases, but its predictive value decreases when moving toward the center of the periodic table. It is incorrect to refer to a sodium chloride (nacl) “molecule” because there is not a single ionic bond, per se, between any specific pair of sodium and chloride ions. That is, group 1 elements form 1+ ions; Web it is important to note, however, that the formula for an ionic compound does not represent the physical arrangement of its ions. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; Click the card to flip 👆.
.PNG)