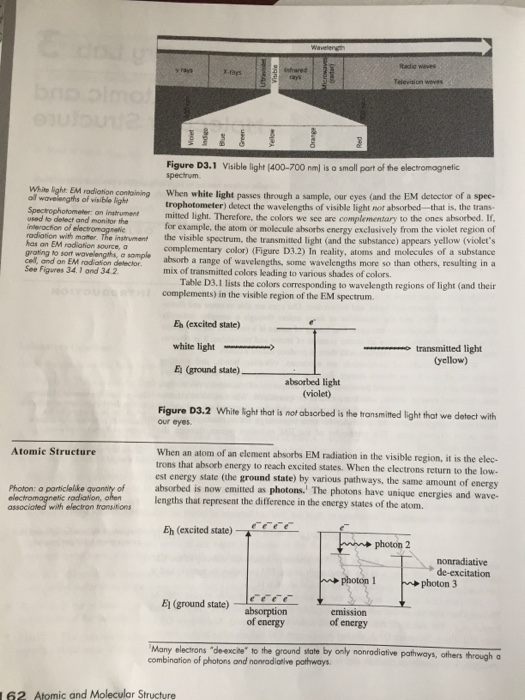
Dry Lab 3 Atomic And Molecular Structure Report Sheet Answers - When appropriate, sample data may be supplied in the lab’s data tables to help you answer the questions. To view and calibrate visible line spectra. Periodic properties note to students: The mercury spectrum instructor's approval of the wavelength grid of the spectra on the color pl b. The mercury spectrum instructor's approval of the wavelength grid of the spectra on the color plate (back cover) b. You are only expected to perform the portions of this lab that do not require you to use laboratory equipment or supplies. The mercury spectrum instructor's approval of the wavelength grid of the spectra on the color plate (back cover): 45% (11) view full document. University of texas, rio grande valley. Web a molecule absorbing incoming energy and going up an excited state what causes a line to appear in a visible line spectrum?
Dry lab 3 report sheet atomic and molecular structure date __________ lab sec. Click the card to flip 👆. The spectra of elements 1. The spectra of elements 1. Dry lab 3 report sheet atomic and molecular structure date a. Dry lab 3 report sheet atomic and molecular structure date lab sec. Web semester 1, unit 3 with extension lab 3: The mercury spectrum instructor's approval of the wavelength grid of the spectra on the color plate (back cover): Click the card to flip 👆. Web a molecule absorbing incoming energy and going up an excited state what causes a line to appear in a visible line spectrum? To identify an element from visible line spectra. 45% (11) view full document. This is a dry lab. The mercury spectrum instructor's approval of the wavelength grid of the spectra on the color plate (back cover) b. To identify a compound from its infrared spectrum. An atom's electron returns to the lowest energy state and the same amount of energy is released as photons When appropriate, sample data may be supplied in the lab’s data tables to help you answer the questions. Dry lab 3 report sheet atomic and molecular structure date lab sec. The spectra of elements 1. Periodic properties note to students: