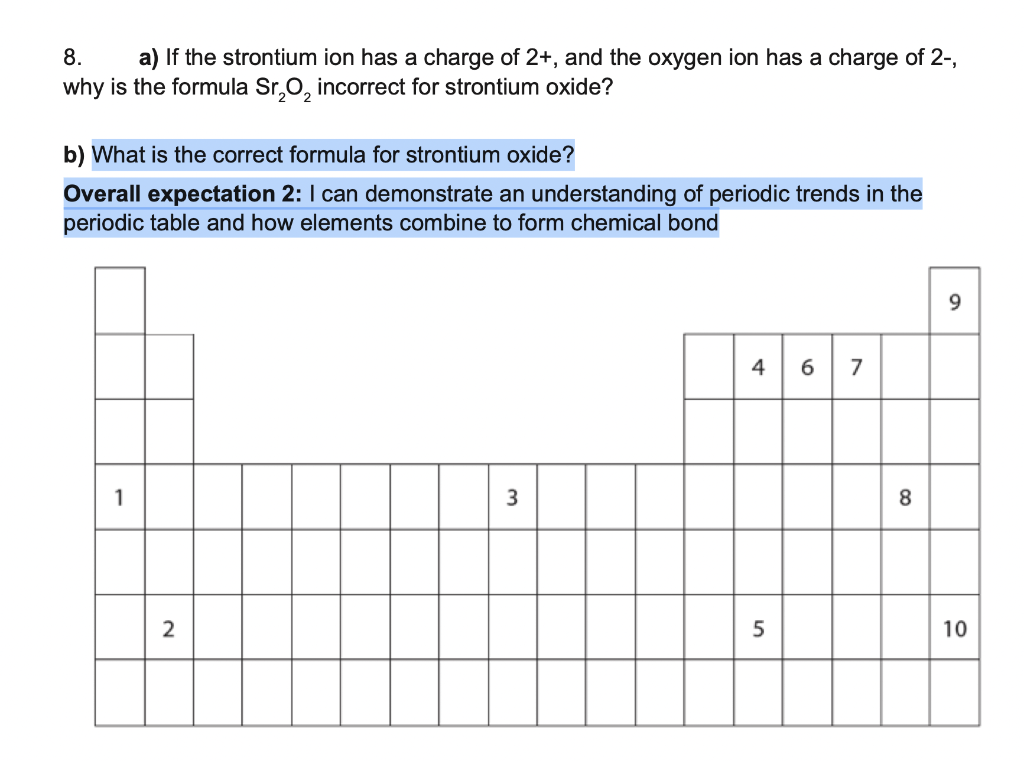
What Is The Charge On The Strontium Ion - Nickel(ii) ni2+ strontium sr2+ zinc zn2+ tin(ii) sn2+. Web the key to writing proper ionic formulas is simple: Because the charges on the ions are characteristic, sometimes we have to have more than one of a cation or an anion to balance the overall positive and negative charges. Al a 3 + aluminum ion: The total positive charge must balance the total negative charge. Using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for. Tin(iv) sn4+ nickel(iv) ni4+ lead(iv) pb4+. Web for each element, write the chemical formula and the name of the ion it forms. For example, iron(ii) has a 2+ charge; Roman numeral notation indicates charge of ion when element commonly forms more than one ion.
Web the key to writing proper ionic formulas is simple: Tin(iv) sn4+ nickel(iv) ni4+ lead(iv) pb4+. Because the charges on the ions are characteristic, sometimes we have to have more than one of a cation or an anion to balance the overall positive and negative charges. Ag a + silver ion Roman numeral notation indicates charge of ion when element commonly forms more than one ion. Using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for. Web the charges of most monatomic ions derived from the main group elements can be predicted by simply looking at the periodic table and counting how many columns an element lies from the extreme left or right. For example, iron(ii) has a 2+ charge; Web for each element, write the chemical formula and the name of the ion it forms. The total positive charge must balance the total negative charge. Nickel(ii) ni2+ strontium sr2+ zinc zn2+ tin(ii) sn2+. 4.1k views 2 years ago. Al a 3 + aluminum ion: Sr a 2 + strontium ion:


.PNG)


.PNG)